About
Bio
FDAnews.com covers domestic and international regulatory, legislative and business news and information for executives in industries regulated by the U.S. Food and Drug Administration, including four newsletters: Drug GMP Report, Drug Industry Daily, International Devices & Diagnostics Monitor and The GMP Letter.
Email
email@cision.one
Website
site@cision.one
Social media




Location
United States of America
Frequency
upgrade
Circulation
upgrade
Sectors
Government Agencies, Pharmaceutical Manufacturers, Pharmacies
Bio
FDAnews.com covers domestic and international regulatory, legislative and business news and information for executives in industries regulated by the U.S. Food and Drug Administration, including four newsletters: Drug GMP Report, Drug Industry Daily, International Devices & Diagnostics Monitor and The GMP Letter.
Website
Social media
Location
Frequency
Circulation
Sectors
Government Agencies, Pharmaceutical Manufacturers, Pharmacies
Most recent articles by FDAnews.com
Lorem ipsum dolor sit amet, consectetur adipiscing elit
Article description
Lorem ipsum dolor sit amet, consectetur adipiscing elit
Article description
Lorem ipsum dolor sit amet, consectetur adipiscing elit
Article description
Explore outlets similar to FDAnews.com
-
NNational Review
Designed as a journal of conservative opinion. Covers the national and international developments related to conservative action and reports and analyzes on what various conservatives are saying, thinking and doing. Committed to providing important, expert analyses of the day’s hottest stories and to breaking those stories in a manner consistent with the publication's worldview.
ViewTThomson Reuters FoundationBackground and Format The Thomson Reuters Foundation, the charitable arm of Thomson Reuters, covers the world’s under-reported stories, with a global team of about 40 staff journalists on humanitarian crises, women’s empowerment, the human impact of climate change, property rights, and trafficking and slavery. All of its stories are published on its news website, news.trust.org, as well as distributed on the Reuters news wire.
ViewPPink Sheet - CitelinePink Sheet is a website delivering insights and lessons learned that biopharma leaders need to inform their decision making and strategic planning from a policy and regulatory perspective, keep themselves and their companies out of trouble and in compliance, and get their products approved. More than just news, Pink Sheet provides analysis, and commentary focused on regulatory implications, including high value perspectives from insiders and thought leaders across the globe – giving regulatory professionals an objective view of how to anticipate challenges, minimize risks, and maximize opportunities. From lessons learned, to why today’s headlines matter; from trends shaping the dynamics of the global pharmaceutical and biotech sectors; to the exclusive, proprietary data behind these trends and more.
ViewSScrip - CitelineScrip, now part of Citeline (a Norstella company), is a premier provider of news, analysis, and data for the global biopharmaceutical industry, offering crucial commercial intelligence on licensing, deals, R&D, market access, and company developments, helping professionals make strategic decisions from drug discovery to market launch.
ViewFFiercePharmaAimed a senior pharmaceutical industry executives, project managers and other business decision makers at pharmaceutical and related life sciences companies, FiercePharma provides daily insider news from the post-approval world including manufacturing, labeling, post-marketing issues and other pharmaceutical industry business topics with a special focus on pharmaceutical company news and the market development of FDA approved products. It includes additional coverage on specific topics such as the changing landscape of generic drugs, pharmaceutical sales and marketing news and manufacturing issues. FiercePharma DOES accept bylined articles/submissions. Contact the editor. The newsletter claims over 150,000 subscribers.
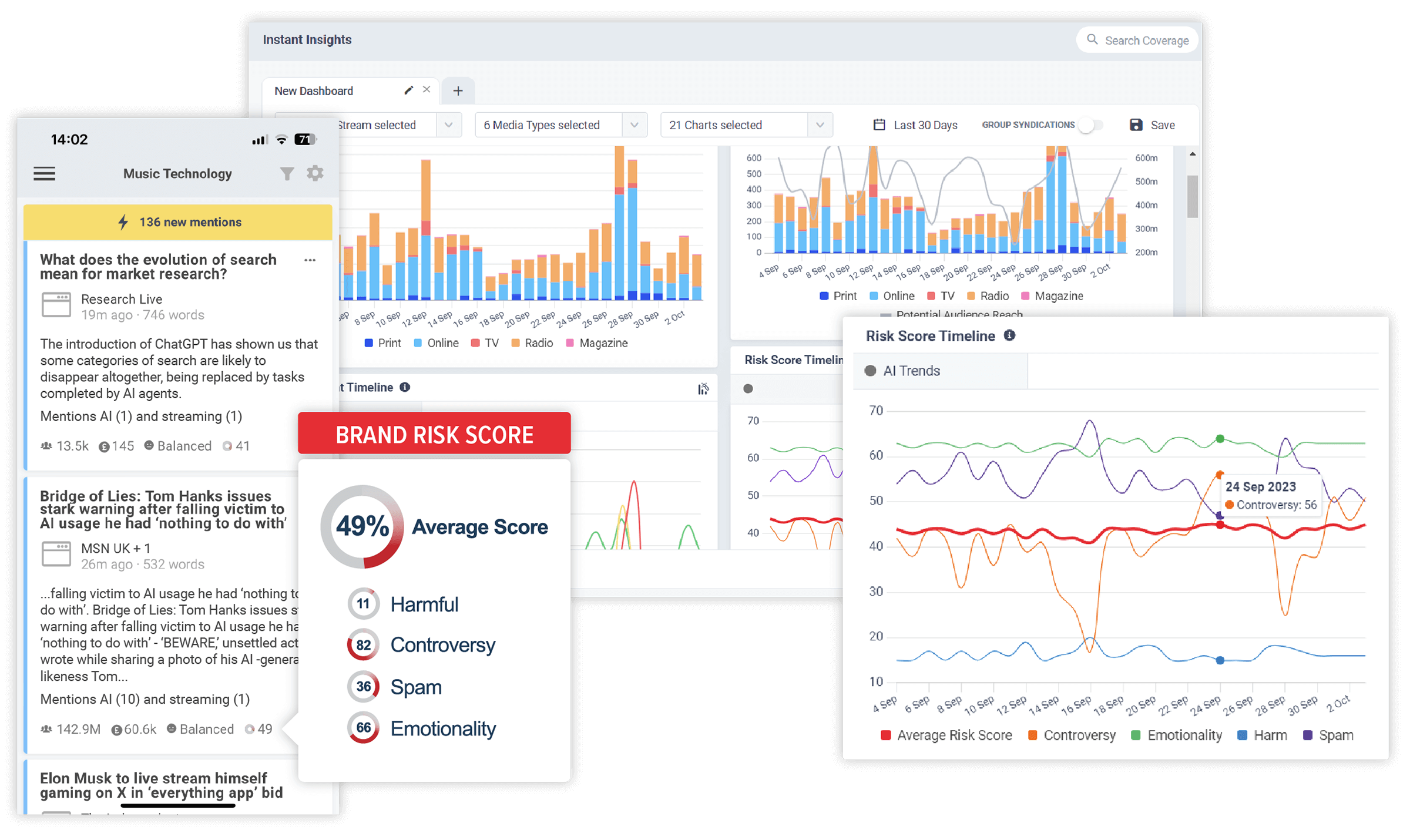
ViewUse CisionOne to find more relevant outletsDiscover the stories that impact your brand. In realtime.
CisionOne delivers relevant news, trends and conversations that matter to your brand with the world’s most comprehensive media monitoring service across Print, Online, TV, Radio, Social, Magazines, Podcasts and more.